 Our CE/FDA-certified MOP.06.6 Flow Diverters demonstrate adherence to the highest international safety, quality, and performance benchmarks. They simplify regulatory compliance and ensure market access for partners worldwide. The global flow diverter market, valued at USD 454.59 million in 2024, shows robust expansion. Experts project a 13.4% Compound Annual Growth Rate from 2025 to 2034, underscoring the critical role of advanced neurovascular solutions.
Our CE/FDA-certified MOP.06.6 Flow Diverters demonstrate adherence to the highest international safety, quality, and performance benchmarks. They simplify regulatory compliance and ensure market access for partners worldwide. The global flow diverter market, valued at USD 454.59 million in 2024, shows robust expansion. Experts project a 13.4% Compound Annual Growth Rate from 2025 to 2034, underscoring the critical role of advanced neurovascular solutions.
Key Takeaways
- MOP.06.6 Flow Diverters are special medical devices. They help treat brain problems like aneurysms. They are safer and work better than old ways.
- These devices have CE and FDA approvals. This means they meet high safety and quality rules. These approvals help them be sold in many countries.
- The company helps partners use these devices. They offer training and support. This makes it easier for hospitals to buy and use them.
Understanding the Role of MOP.06.6 Flow Diverters in Neurovascular Treatment

The Critical Importance of Flow Diverters
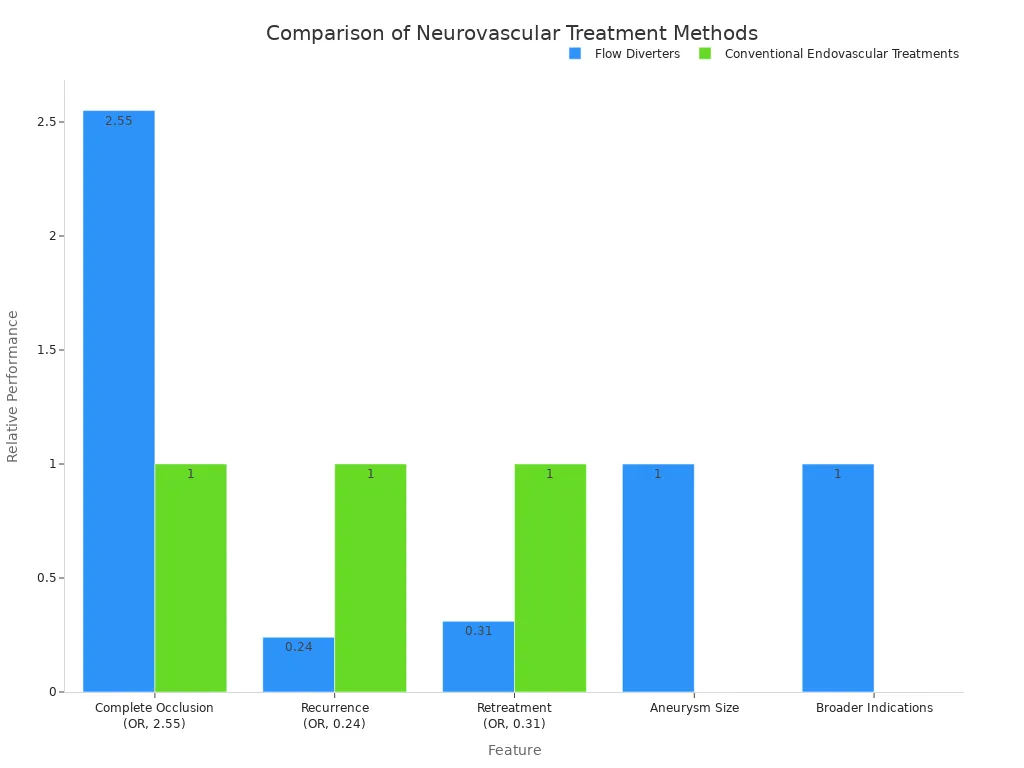
Flow diverters represent a monumental leap in neurovascular treatment. They offer a less invasive and highly effective solution for complex conditions. These innovative devices are crucial for treating various neurovascular issues. This includes intracranial aneurysms, aneurysms in the internal carotid artery (ICA), and larger, wide-neck aneurysms. Flow diverters provide significant advantages over traditional methods. They safely cover most branch vessels, including ophthalmic, posterior communicating, and anterior choroidal arteries. Vision outcomes for paraclinoid aneurysms also show improvement with flow diverters. Patients experience better vision and less harm compared to clipping or coiling. Long-term stability is a key benefit. Complications six months after treatment are rare. Recanalization after occlusion is also exceedingly rare. The efficacy rates approach 95%, demonstrating superior results. This good safety profile comes from avoiding direct manipulation of the aneurysm itself.
| Feature | Flow Diverters (FDs) | Conventional Endovascular Treatments |
|---|---|---|
| Complete Occlusion | Higher rate (OR, 2.55) | Lower rate |
| Recurrence | Lower rate (OR, 0.24) | Higher rate |
| Retreatment | Lower rate (OR, 0.31) | Higher rate |
| Aneurysm Size | Larger size | Smaller size |
| Broader Indications | Yes | No |
Navigating Global Medical Device Procurement
Procuring medical devices globally presents unique challenges. Organizations face constant changes in regulatory compliance. They must stay updated with standards from bodies like the FDA and EMA. Maintaining consistent high-quality standards across all production batches is also vital. This requires robust Quality Management Systems. Supply chain disruptions can interrupt material supply. Companies need supplier diversification and clear supply chain visibility. Adapting to new technologies during production also demands agile processes. Protecting innovations through patents is essential in a competitive industry. Entering new markets requires overcoming compliance standards and competition. Thorough market research becomes crucial. Medical device cybersecurity risks increase as devices become more connected. Validating device security protects healthcare systems and data. The MOP.06.6 FLOW DIVERTER addresses these complexities. It offers a reliable solution for global procurement teams.
The Gold Standard: CE and FDA Certifications for MOP.06.6 Flow Diverters
CE Marking: Gateway to the European Market CE marking opens the door to the vast European market. For Class III medical devices, like our MOP.06.6 FLOW DIVERTER, the conformity assessment route is rigorous. It involves consultation, referencing specific directives. A clinical evaluation consultation procedure is also necessary. Manufacturers must issue a declaration of conformity and affix the CE Mark. Clinical investigations are generally required for these devices. Exceptions exist if sufficient clinical data already exists for equivalent devices. This also applies if the manufacturer has conducted Post-Market Clinical Follow-up (PMCF) studies. The declaration of conformity must have support from a notified body assessment. An expert panel consultation follows this. The conformity assessment process typically ranges from 9 to 24 months. However, this timeline can extend due to device novelty or complexity.
FDA Approval: Benchmark for the United States Market
FDA approval sets the benchmark for the United States market. For Class III medical devices, Premarket Approval (PMA) is essential. The PMA application must include general information, such as the applicant’s name and address. It also requires a 10-15 page summary of all application data. A thorough description of the device and its functional components is crucial. Details on manufacturing methods, facilities, and controls are also necessary. The application must include a bibliography of all published reports. Copies of all proposed labeling are also a requirement. Technical sections covering pre-clinical and clinical studies are particularly important.
The Synergistic Value of Dual Certification
Dual CE and FDA certification offers immense synergistic value. It demonstrates an unwavering commitment to global safety and quality standards. This dual approval streamlines market access across two major economic blocs. It also builds profound trust among healthcare providers and procurement partners. This comprehensive validation assures stakeholders of the device’s efficacy and reliability. It simplifies regulatory navigation for international trade.
Meeting Global B2B Procurement Standards with CE/FDA-Certified MOP.06.6 Flow Diverters
Ensuring Regulatory Compliance and Market Access
Navigating the complex world of medical device regulations can be challenging. Many teams often delay considering regulatory issues until late in the design process. This can lead to costly redesigns and impact sales revenue. Some common pitfalls include skipping the Design History File (DHF) or underestimating risk management. Others forget post-market surveillance (PMS) or treat usability as an afterthought. Our CE/FDA-certified MOP.06.6 FLOW DIVERTER helps partners avoid these issues. These certifications mean the device already meets stringent requirements. This simplifies market entry into Europe and the United States. It also provides a strong foundation for global expansion. We ensure comprehensive documentation and early integration of regulatory considerations. This proactive approach saves time and resources for our partners.
Demonstrating Uncompromising Quality and Safety
Quality and safety stand as the cornerstones of medical device procurement. Our commitment to these principles shines through our certifications. We adhere to international standards like ISO 13485, which specifically outlines requirements for medical device quality management systems. This standard builds upon ISO 9001, adding unique demands for the medical device sector. It includes specific documentation for device files, controls for the work environment, and production requirements for cleanliness. ISO 14971 also works with ISO 13485, providing detailed requirements for risk management. These rigorous systems ensure consistent product quality and reliability. They minimize the risk of safety incidents, such as stroke or intracranial hemorrhage, which are critical concerns in neurovascular procedures. Our certifications provide tangible proof of our dedication to patient well-being and product excellence.
Facilitating Due Diligence and Risk Mitigation
B2B medical procurement teams conduct thorough due diligence for new suppliers. They assess financial stability, requiring years of financial accounts and demonstrating positive net asset value. Certifications and standards, such as ISO 9001, ISO 14001, and ISO 27001, are also crucial. Our CE and FDA certifications significantly reduce perceived risk for procurement partners. These approvals assure patients and healthcare providers of a product’s safety and effectiveness. They minimize defects and failures that could harm users. This dual certification also lowers liability risks for healthcare providers. It builds confidence among both patients and medical professionals. Our comprehensive compliance frameworks and robust risk assessments streamline this process. We provide all necessary documentation, ensuring transparency and trust.
Supporting Clinical Efficacy and Physician Confidence
Physicians need confidence in the devices they use for patient care. Our CE/FDA certifications provide this assurance. These approvals are more than just regulatory hurdles; they are key determinants in medical device procurement. They guarantee patient safety, product reliability, and adherence to regulations. This comprehensive validation assures stakeholders of the device’s efficacy. It confirms the device performs as expected in clinical settings. This builds profound trust among healthcare providers. They know the device has undergone rigorous testing and evaluation. This confidence translates into better patient outcomes and greater peace of mind for medical professionals.
Streamlining Supply Chain and International Trade
Global supply chains can be complex, with varying regulatory landscapes. Our CE/FDA-certified devices simplify international trade for our partners. These certifications mean the product already meets the highest global standards. This reduces the need for additional, country-specific approvals in many regions. It streamlines logistics and customs processes. Procurement managers, hospitals, and distributors can rely on these certifications. They ensure a smoother flow of goods across borders. This efficiency helps maintain consistent product availability. It also reduces administrative burdens and potential delays. Our commitment to these global standards makes us a reliable partner in the international medical device market.
Beyond Certification: Our Commitment to Procurement Partners for MOP.06.6 Flow Diverters
Continuous Quality Improvement and Post-Market Surveillance
Certifications mark a beginning, not an end. We actively pursue continuous quality improvement. Our robust post-market surveillance (PMS) system ensures ongoing safety and efficacy. This system adheres to strict global regulations. For instance, FDA 21 CFR Part 822 outlines procedures for high-risk Class II and III devices. It requires a surveillance plan, data collection, and periodic reporting. ISO 13485, our quality management standard, mandates feedback systems, complaint handling, and internal audits. Furthermore, EU MDR (Regulation (EU) 2017/745) introduces comprehensive PMS requirements. These include a PMS Plan, a Periodic Safety Update Report (PSUR), and Post-Market Clinical Follow-Up (PMCF). We use this data to update risk assessments and refine designs. This proactive approach ensures our devices consistently meet the highest standards.
Dedicated Support for Global Procurement Teams
We believe in strong partnerships. Our commitment extends beyond product delivery. We offer dedicated support to global procurement teams. This includes comprehensive training programs. These programs empower our partners with essential knowledge. For example, we provide training on FDA regulatory compliance and quality principles. We also offer insights into auditing and qualifying suppliers. Our experts guide partners through complex regulatory landscapes. They help streamline procurement processes. This collaborative approach fosters mutual growth and success. We stand ready to assist our partners at every step. Together, we achieve excellence in neurovascular care.
The CE/FDA-certified MOP.06.6 FLOW DIVERTER seamlessly integrates into global B2B medical procurement standards. It consistently exceeds these benchmarks. This device offers unparalleled safety, efficacy, and regulatory confidence. Partners worldwide benefit from its proven quality. We empower them to achieve excellence in neurovascular care.
FAQ
What makes CE and FDA certifications vital for the MOP.06.6 FLOW DIVERTER?
These certifications confirm the MOP.06.6 FLOW DIVERTER meets global safety and quality standards. They simplify market access and build profound trust among healthcare providers.






